This web page was produced as an assignment for Genetics 677, an undergraduate course at UW-Madison.
Scientific Article Review
Introduction
The authors begin with a review of the phenotypic characterizations (manic and depressive states) of bipolar disorder (BDP). They discuss recent literature that found evidence supporting the hypothesis that BPD is a complex disease that arises from inheritance of multiple susceptibility genes. Recent human genetic studies have identified GRIK2, which encodes for GluR6, as a potential BPD susceptibility gene. Genetic linkage of BPD to chromosome 6q21 has been demonstrated in several studies, and genome-wide significant linkage was recently established by meta-analysis. A specific haplotype located on the regulatory region of the human GluR6 gene was found to be associated with BPD. Furthermore, post-mortem studies demonstrate a reduction in GluR6 mRNA levels in the brains of BPD patients.
Further rationale for the implication of GRIK2 as one of the BPD susceptibility genes is the expression of kainate receptors in the areas of neuronal circuits involved in mood regulation. They have been shown to bidirectionally regulate the release of glutamate, and to be involved in long-term potentiation induction at the hippocampus and amygdala.
Study Questions
1. Undertook a series of animal studies with mice to elucidate the role of this receptor (GRIK2) in modulating behavioral patterns related to phenotypic patterns of the complex systems of BPD, using a series of behavioral tests tailored to monitor these diverse symptoms of BPD.
2. To determine potential specificity of this receptor to mood-related behaviors, they investigated the role of GluR5 receptors under the same series of behavioral tests.
3. The effects of lithium (the classic antimanic mood stabilizer) on behavioral alterations of mutant mice (GluR6 KO) were investigated.
4. To assess any potential biological changes associated with GluR6 ablation or a possible biochemical mechanism mediating abberant behaviors in GluR6 KO mice, a series of biochemical tests were conducted.
Study System & Experimental Tests
GluR6 and GluR5 knockout (KO) male mice and wildtype male mice, aged 8 weeks old, were used for these experiments. Female mice were not used in the study to avoid confounding effects of the menstrual cycle on behavioral and neurochemical tests. All tests were performed at least 24 hours apart, and were performed in an order from less to more intrusive. Additional cohorts of mice were used for the passive avoidance test, the lithium treatment experiments, and the amphetamine treatment experiments.
The experimental tests included: a passive-avoidance test, an open-field test, monitoring home cage activity, a social interaction test, an elevated plus-maze test, a forced swim test, a resident intruder test, and immunoblotting. Several of these behavioral tests were given additionally with cohorts of mice treated with lithium, and cohorts of mice treated with amphetamine.
Passive-Avoidance Test
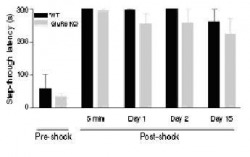
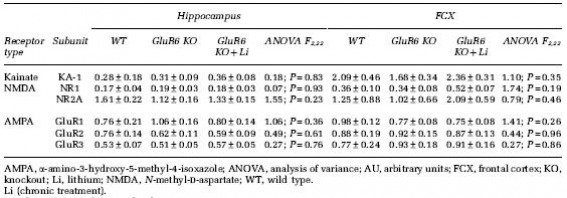
Figure 1: GluR6 mice have no significant impairment in the passive avoidance test. This test assesses fear-associated learning and memory. No significant effects of genotype between the GluR6 KO mice and WT mice were detected. During the pre-shock training trial, all mice entered the dark compartment quickly. After receiving foot shocks in the dark compartment during this trial, all mice quickly learned to avoid entering the dark compartment, as indicated by the increased latencies during the post-shock test trials of 5 minutes, 1 day, 2 days, and 15 days. No statistical significance, however, was found among the post-shock avoidance latencies.
GluR6 KO mice exhibited more spontaneous activity
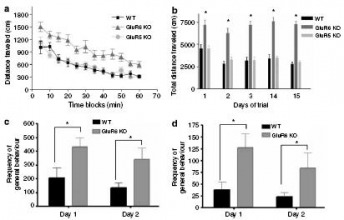
Figure 2: GluR6 KO mice exhibit increased spontaneous activity in the open field and home cage.
(a) Open-field test 1: Mice were placed in one corner of an open-field arena and the distance traveled was recorded for 60 minutes. GluR6 KO mice traveled significantly farther in this test than the WT and GluR5 mice.
(b) Open-field test 2: Again, mice were placed in one corner of an open-field arena and the distance traveled was recorded. This time, however, the persistence of this behavior was assessed by recording the distance traveled over 3 consecutive days, and at days 14 and 15 from the first exposure. Again, GluR6 KO mice traveled significantly farther in this test than the WT and GluR5 mice.
(c) Home-cage activity test 1: Mice were monitored in their home cages using a digital camcorder for 1 hour for 2 consecutive days. The behavior monitored was that of general activity, including turning, walking, digging, grooming, drinking, and eating. GluR6 mice displayed significantly more home cage general activity, which was consistent over two days of testing.
(d) Home-cage activity test 2: Again, mice were monitored in their home cages using a digital camcorder for 1 hour for 2 consecutive days. The behavior monitored was exploratory behavior. GluR6 mice displayed significantly more home cage exploratory behavior activity, which was again consistent over two days of testing.
GluR6 mice were more responsive to amphetamine
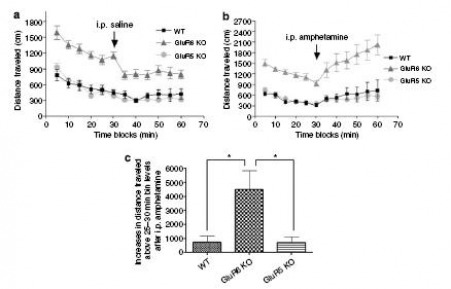
Figure 3: GluR6 KO mice exhibit increased amphetamine-induced response.
(a) Control test for injection-induced irritability. Mice were placed in one corner of the arena and their behavior observed for 30 minutes, then injected with saline, and then placed back in the same arena for another 30 minutes and their behavior recorded. Saline injection did not affect locomotor activity for any the three groups.
(b & c) Amphetamine-induced hyperactivity test: Same procedure as in (a), only with an amphetamine injection instead of a saline injection. Amphetamine injection increased locomotor activity for all three genotypes above the activity level before the injection. The amphetamine-induced increases in locomotion were significantly higher in GluR6 KO mice above the 25-30 minute activity levels.
GluR6 KO mice were robustly aggressive
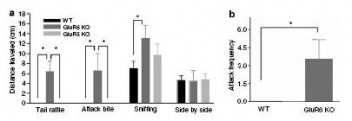
Figure 4: GluR6 mice display robustly aggressive behavior.
(a) Social interaction test: Pairs of mice were placed in opposite corners of an open-field arena and the frequency of social behaviors (including tail rattle, attack bite, sniffing, and side-by-side) was counted over 5 minutes. GluR6 KO mice displayed a higher frequency of aggressive behaviors (tail rattle, attack bite) and also of exploratory behaviors (sniffing) than GluR5 KO and WT mice.
(b) Resident-intruder test: A naive male mouse was introduced to the home cage of each mouse tested. The frequency of attacks on the intruder by the resident mouse was counted over a 5 minute period. While WT mice exhibited no attacks towards an intruder, GluR6 KO mice exhibited a high frequency of attacks toward their intruder.
GluR6 KO mice exhibited less anxious and more risk-taking behavior
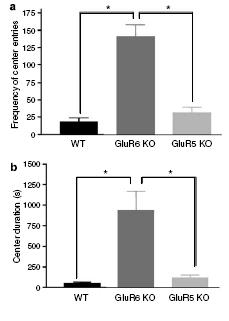
Figure 5: GluR6 KO mice show increased activity in anxiety-provoking subregions of the open-field test.
(a) Open-field test (Entry into arena center): Mice were placed in one corner of an open-field arena, and the frequency of entering the center of the arena (an anxiety-provoking region) was counted. GluR6 KO mice displayed a higher frequency of entries to the center of the open-field arean compared with GluR5 KO or WT mice.
(b) Open-field test (Duration spent in arena center): Mice were placed in one corner of an open-field arena, and the total time spent in the center of the arena (an anxiety-provoking region) was recorded over 60 minutes. GluR6 KO mice increased their time spent in the center of the open-field arena compared with GluR5 KO or WT mice.

Figure 6: GluR6 KO mice exhibited increased activity in anxiety-provoking regions of the elevated plus maze test.
(a) Ratios of open-arm v. total duration of time spent: Each mouse was placed in the center of a plus-shaped maze elevated above ground containing two dark closed arms and two open and lit arms without walls. GluR6 KO mice exhibited increases in duration of time spent in the open arms of the maze (anxiety-provoking region) over a 5 minute period, compared to GluR5 KO mice and WT mice.
(b) Ratios of open-arm v. total arm entries: Each mouse was placed in the same maze as in (a). GluR6 KO mice exhibited increased open-arm entries (anxiety-provoking regions) over a 5 minute period, compared to GluR5 KO mice and WT mice.
(c) Ratios of open-arm v. total arm distance traveled: Each mouse was placed in the same maze as in (a). GluR6 KO mice exhibited increased distance traveled in the open arms (anxiety-provoking regions) over a 5 minute period, compared to GluR5 KO mice and WT mice.
GluR6 KO mice displayed less immobility in the forced swim test
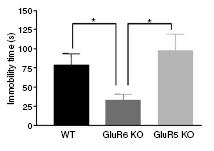
Figure 7: GluR6 mice displayed decreased immobility time in the forced swim test. Each mouse was placed in cylinders for 6 minutes filled with water in such a way that the mouse was not able to touch the floor or escape using the walls. Immobility time was measured throughout the last 4 minute of the session, and was defined as a lack of activity apart from small movements necessary to keep the body floating, as opposed to behavioral escape attempts. Immobility time was reduced for GluR6 KO mice compared with GluR5 KO or WT mice.
Behavioral effects of lithium treatment on GluR6 KO and WT mice
Lithium Treatment: Reduced spontaneous locomotor activity
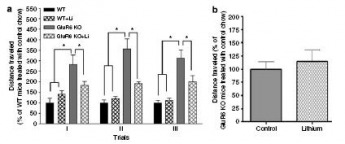
Figure 8: Chronic lithium treatment (introduced into food) reduced spontaneous locomotor activity in GluR6 KO mice.
(a) Open-field test (long-term): Over four weeks of lithium treatment (trials I-III), the spontaneous locomotor activity, measured by distance traveled, of GluR6 KO mice studied over 3 consecutive days was reduced compared to the WT mice.
(b) Open-field test (short-term): 4 days of lithium treatment did not significantly alter the spontaneous locomotor activity of GluR6 KO mice compared with WT mice.
Lithium Treatment: Decreased aggressive behavior
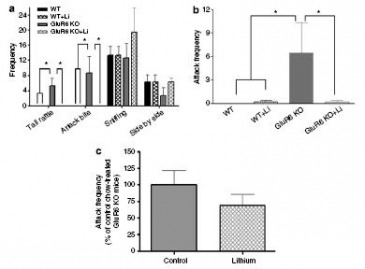
Figure 9: Chronic lithium treatment (long-term) dramatically decreased aggressive behavior in GluR6 KO mice.
(a) Social Interaction test: 4 weeks of lithium treatment resulted in a complete loss of aggressive behavior (tail rattling and attack bite) in GluR6 KO mice, compared to WT mice. The other non-aggressive behaviors remained the same.
(b) Resident-Intruder test (4 weeks): 4 weeks of lithium treatment resulted in a robust reduction in attacks on naive intruder mice displayed by resident GluR6 KO mice, as compared to WT mice.
(c) Resident-Intruder test (short-term): 6 days of lithium treatment did not significantly reduce attack frequencies of GluR6 KO mice, as compared to WT mice.
Lithium Treatment: Decreased activity in anxiety-provoking regions
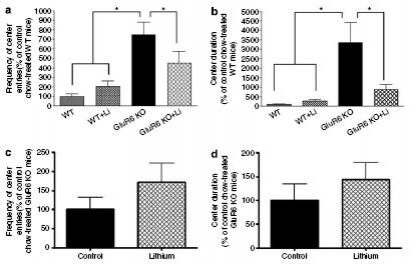
Figure 10: Chronic lithium treatment attenuated increased activity in anxiety-provoking regions.
(a) open-field test (entries/long-term): 4 weeks of lithium treatment reduced the frequency of entries to the center of the open-field arena (anxiety-provoking region) during the 1st day of testing in GluR6 KO mice, as compared to WT mice.
(b) open-field test (duration/long-term): 4 weeks of lithium treatment reduced the duration of time spent in the center of the open-field arena (anxiety-provoking region) during the 1st day of testing in GluR6 KO mice, as compared to WT mice.
(c) open-field test (entries/short-term): 5 days of lithium treatment did not reduce the frequency of center entries of GluR6 KO mice.
(d) open-field test (duration/short-term): 5 days of lithium treatment did not reduce the duration of time spent in the arena center of GluR6 KO mice.
Lithium Treatment: Effect on elevated plus maze test (anxiety-provoking regions)
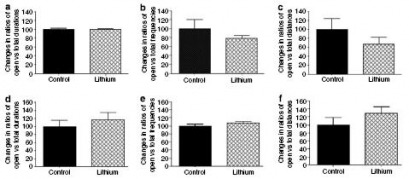
Figure 11: Effects of chronic and short-term lithium treatment on elevated plus maze test results.
(a-c): Chronic (4 weeks) lithium treatment: No significant alteration in GluR6 KO mice of (a) duration of time spent in the open arms of the maze, (b) frequency of open-arm entries, or (c) distance draveled in the open arms.
(d-f): Short-term (4 days) lithium treatment: No significant alteration in GluR6 KO mice of (a) duration of time spent in the open arms of the maze, (b) frequency of open-arm entries, or (c) distance draveled in the open arms.
Lithium Treatment: Reduced immobility time in forced swim test
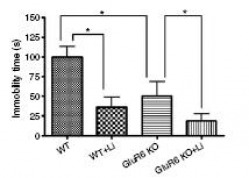
Figure 12: Chronic lithium treatment reduced both GluR6 KO and WT mice immobility time during the forced swim test. The GluR6 KO mice, however, exhibited less immobility compared to the WT mice.
Effects of ablation of GluR6 and chronic lithium treatment on cell membrane levels of ionotropic glutamatergic receptors
GluR5 subunit membrane expression levels
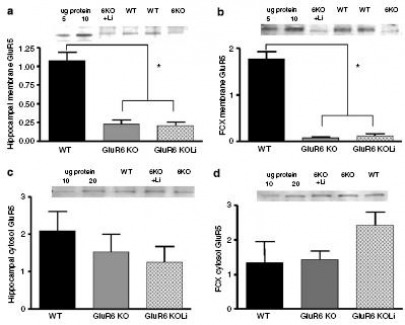
Figure 13: The GluR5 subunit membrane expression levels were reduced in the hippocampus and FCX of GluR6 KO mice.
(a) Hippocampal membrane GluR5: The GluR5 protein levels were reduced in the hippocampal membrane fraction of the GluR6 KO mice. 4 weeks of chronic lithium treatment did not affect GluR5 protein level reduction.
(b) FCX membrane GluR5: The GluR5 protein levels were reduced in the FCX membrane fraction of the GluR6 KO mice. 4 weeks of chronic lithium treatment did not affect GluR5 protein level reduction.
(c) Hippocampal cytosol GluR5: The GluR5 protein levels were not reduced in the hippocampal cytosol fraction of the GluR6 KO mice. 4 weeks of chronic lithium treatment did not affect GluR5 protein level reduction.
(d) FCX cystosol GluR5: The GluR5 protein levels were not reduced in the FCX cytosol fraction of the GluR6 KO mice. 4 weeks of chronic lithium treatment did not affect GluR5 protein level reduction.
KA2 subunit membrane expression levels
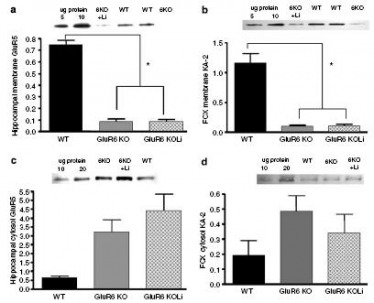
Figure 14: KA-2 subunit membrane expression levels were reduced in the hippocampus and FCX of GluR6 KO mice.
(a) Hippocampal membrane GluR5: The KA-2 protein levels were reduced in the hippocampal membrane fraction of GluR6 KO mice. 4 weeks of chronic lithium treatment did not affect KA-2 protein level reduction.
(b) FCX membrane KA-2: The KA-2 protein levels were reduced in the FCX membrane fraction of GluR6 KO mice. 4 weeks of chronic lithium treatment did not affect KA-2 protein level reduction.
(c) Hippocampal cytosol GluR5: A trend was observed toward increased KA-2 protein levels in the hippocampal cytosol fraction of GluR6 KO mice. This trend was not affected by lithium treatment.
(d) FCX cytosol KA-2: A trend was observed toward increased KA-2 protein levels in the FCX cytosol fraction of GluR6 KO mice. This trend was not affected by lithium treatment.
Ionotropic glutamate receptor subunits membrane expression levels
Table 1: Ionotopic glutamate receptor subunits membrane expression levels (KARs subunit KA-1, the N-methyl-D-aspartate (NMDA) receptor subunits NR1 and NR2A, and the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole (AMPA) receptor subunits GluR1, GluR2, and GluR3), are not altered in the hippocampus or FCX of GluR6 KO mice, and are not affected by short-term or chronic lithium treatment.
Conclusions
This study was the first to demonstrate with repeated biochemical and behavioral tests that GluR6, a KAR (kainate receptor) subunit, plays a critical role in the general control of diverse behavioral features, including hyperactivity, drive, aggressiveness, risk taking, less anxious behavior, and supersensitivity to psychostimulants like amphetamines. GluR6 KO mice displayed these behavioral features without any other mental impairment, GluR6 ablation was associated with reduced membrane GluR5 or KA-2 receptors, GluR5 ablation was completely without affect in any of the tested behaviors, and membrane levels of other ionotropic glutamatergic receptors remained unchanged in the hippocampus and FCX of GluR6 KO mice. Taken together, these results indicate that GluR6 seems to be uniquely able to control a subset of BPD behavioral manifestations in this mouse model. Futher investigation is needed to discover whether GluR6 is only involved in the control mechanisms of manic symptoms, or whether it is also involved in the pathology of genetic susceptibility to BPD.
References:
1. Shaltiel, G., S. Maeng, O. Malkesman, B. Pearson, R. J. Schloesser, T. Tragon, M. Rogawski, M. Gasior, D. Luckenbaugh, G. Chen, and H. K. Manji. "Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania." Molecular Psychiatry 13 (2008): 858-72.
| shaltieletal2008.pdf | |
| File Size: | 512 kb |
| File Type: | |
Ashley Bateman, [email protected], last updated 5/13/09
Genetics 677